Orphan Drug Market Projected to Reach USD 443.77 Billion by 2030
Innovative Technologies and Regulatory Incentives Drive Significant Growth
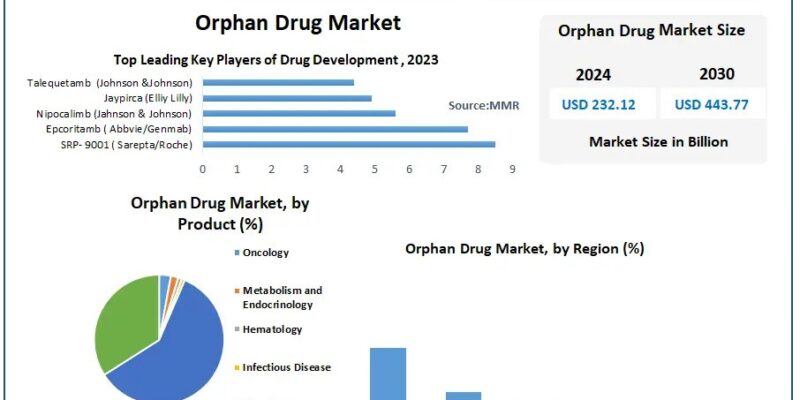
The Orphan Drug Market Growth is poised for substantial expansion, with projections indicating a rise from USD 232.12 billion in 2023 to approximately USD 443.77 billion by 2030, reflecting a compound annual growth rate (CAGR) of 9.7%.
Request a Free Sample of the Report for Detailed Insights! https://www.maximizemarketresearch.com/request-sample/342/
Market Definition and Overview
Orphan drugs are pharmaceutical agents specifically developed to diagnose, prevent, or treat rare diseases or conditions, often referred to as orphan diseases. In the United States, a rare disease is defined as one affecting fewer than 200,000 individuals. The development of orphan drugs is incentivized by legislation such as the Orphan Drug Act (ODA) of 1983, which offers benefits like tax credits, grant funding, and market exclusivity to encourage pharmaceutical companies to invest in treatments for rare diseases.
Market Growth Drivers and Opportunities
Several key factors are propelling the growth of the orphan drug market:
-
Emerging Technologies: Advancements in gene editing, artificial intelligence (AI), and novel drug delivery systems are revolutionizing the development of orphan drugs. Technologies such as CRISPR gene editing and AI-driven drug discovery are enabling more precise and efficient targeting of genetic mutations responsible for rare diseases. For instance, in 2023, an AI-driven biotech company received the FDA’s inaugural Orphan Drug Designation for INS018_055, a small molecule inhibitor for treating idiopathic pulmonary fibrosis.
-
Regulatory Incentives: Governments worldwide have implemented policies to encourage the development of orphan drugs. In the United States, the ODA provides benefits such as tax breaks, grant funding, and seven years of market exclusivity upon approval. These incentives have significantly increased the number of orphan drug approvals, with the FDA approving over 500 orphan drugs in the decade following the ODA’s enactment.
-
Rising Prevalence of Rare Diseases: Increased awareness and improved diagnostic techniques have led to the identification of more rare diseases, expanding the target population for orphan drugs. This heightened awareness has also spurred patient advocacy and funding for research into rare conditions.
-
High Return on Investment: Despite smaller patient populations, orphan drugs often command premium pricing due to the lack of alternative treatments and the high value placed on addressing unmet medical needs. This pricing power has made orphan drugs an economically viable strategy for biopharmaceutical companies, with the orphan drug market exhibiting a compound annual growth rate (CAGR) of 25.8% between 2001 and 2011, compared to 20.1% for non-orphan drugs.
Claim Your Free Sample to Access the Full Report! https://www.maximizemarketresearch.com/request-sample/342/
Market Segmentation Analysis
The orphan drug market can be segmented based on disease type, product type, and region.
1. Disease Type
-
Oncology: A significant portion of orphan drugs is dedicated to rare cancers, addressing conditions such as acute lymphoblastic leukemia and multiple myeloma. The high unmet need in oncology has driven substantial investment in this segment.
-
Metabolic Disorders: Orphan drugs targeting metabolic disorders, such as Gaucher disease and Fabry disease, have seen notable advancements, offering patients therapeutic options where none previously existed.
-
Neurological Disorders: Rare neurological conditions, including Huntington’s disease and amyotrophic lateral sclerosis (ALS), have been focal points for orphan drug development, leading to innovative treatments that improve patient outcomes.
2. Product Type
-
Biologics: Biological products, including monoclonal antibodies and gene therapies, constitute a significant segment of the orphan drug market due to their effectiveness in targeting specific disease mechanisms.
-
Small Molecule Drugs: Traditional chemical compounds continue to play a crucial role, especially in cases where oral administration and systemic effects are desired.
3. Distribution Channel
-
Hospital Pharmacies: Given the specialized nature of many orphan drugs, hospital pharmacies are primary distribution channels, ensuring proper administration and monitoring.
-
Retail Pharmacies: For certain orphan drugs, especially those administered orally, retail pharmacies provide accessibility to patients, enhancing treatment adherence.
Looking for More Information? Explore Further Details Here! https://www.maximizemarketresearch.com/market-report/orphan-drugs-market/342/
Regional Analysis
North America
North America, particularly the United States, holds a dominant position in the orphan drug market. The presence of robust healthcare infrastructure, favorable regulatory frameworks like the ODA, and significant investment in research and development contribute to this leadership. The U.S. market’s emphasis on innovation and early adoption of advanced therapies further propels growth.
Europe
Europe represents a substantial share of the orphan drug market, supported by initiatives such as the European Union’s Orphan Medicinal Products Regulation, which offers incentives similar to those in the U.S. Countries like Germany, France, and the United Kingdom have established frameworks to encourage orphan drug development, leading to increased availability of treatments for rare diseases.
Asia-Pacific
The Asia-Pacific region is experiencing rapid growth in the orphan drug market, driven by increasing healthcare expenditure, improved diagnostic capabilities, and growing awareness of rare diseases. Countries such as Japan and China are implementing policies to support orphan drug development, recognizing the importance of addressing unmet medical needs within their populations.
Competitive Landscape
The orphan drug market is characterized by the presence of both large pharmaceutical companies and specialized biotechnology firms. Key players include:
-
Alexion Pharmaceuticals, Inc.: Known for Soliris, a treatment for paroxysmal nocturnal hemoglobinuria, Alexion has established itself as a leader in the orphan drug space.
-
Biogen: With products like Spinraza for spinal muscular atrophy, Biogen demonstrates a strong commitment to neurological orphan diseases.
-
Vertex Pharmaceuticals: Developer of CFTR modulators for cystic fibrosis, Vertex has made significant strides in treating this rare genetic disorder.
To explore More Reports, visit our website:
Global Tonometer Market https://www.maximizemarketresearch.com/market-report/global-tonometer-market/110354/
Lupus Nephritis Market https://www.maximizemarketresearch.com/market-report/lupus-nephritis-market/187839/
Face Mask Market https://www.maximizemarketresearch.com/market-report/face-mask-market/157665/
Global Therapeutic Vaccines Market https://www.maximizemarketresearch.com/market-report/global-therapeutic-vaccines-market/25494/
About Maximize Market Research:
Maximize Market Research is a versatile market research and consulting firm with expertise across a wide range of industries. Our coverage includes medical devices, pharmaceutical manufacturing, science and engineering, electronic components, industrial equipment, technology and communication, automotive, chemicals, general merchandise, beverages, personal care, and automated systems, among others. We offer a comprehensive suite of services, including market-validated industry estimates, technical trend analysis, in-depth market research, strategic consulting, competitive analysis, production and demand evaluation, and client impact studies.
Contact Maximize Market Research:
Address :
3rd Floor, Navale IT Park, Phase 2
Pune-Bangalore Highway, Narhe
Pune, Maharashtra 411041, India
Email: sales@maximizemarketresearch.com
Phone: +91 96071 95908, +91 9607365656