Global Single-use Medical Device Reprocessing Industry: Key Statistics and Insights in 2024-2032
Summary:
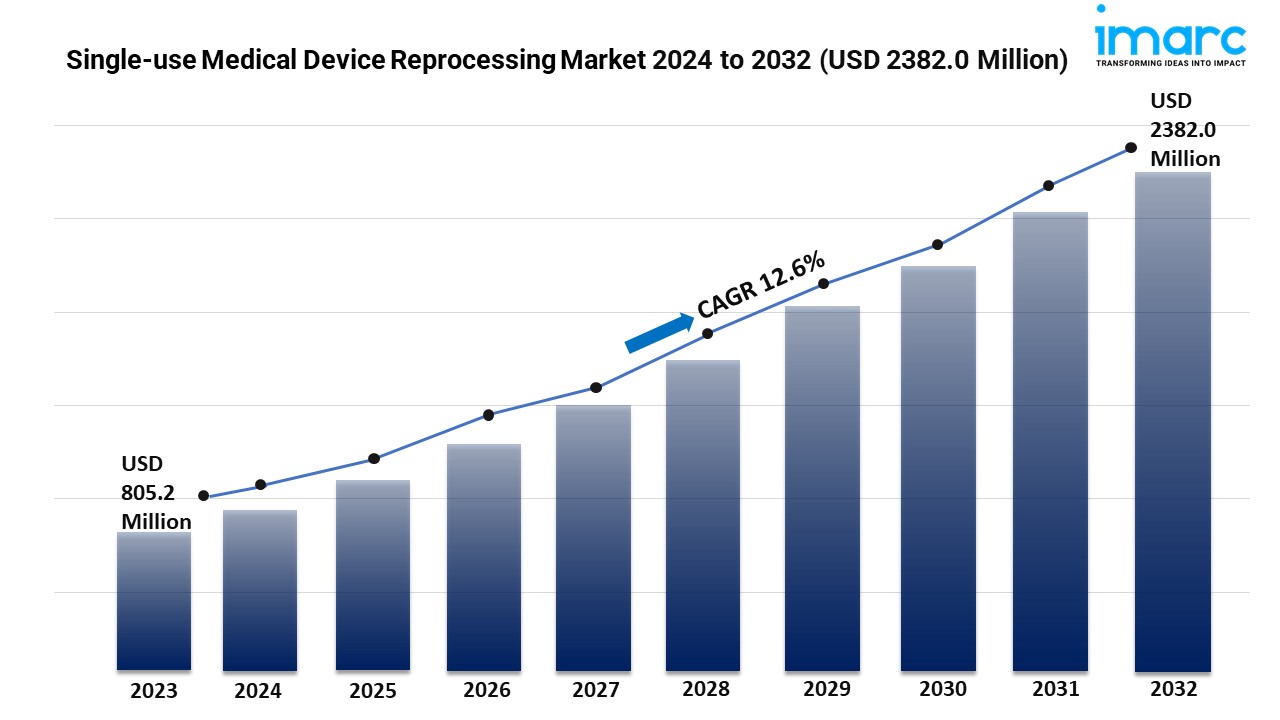
- The global single-use medical device reprocessing market size reached USD 805.2 Million in 2023.
- The market is expected to reach USD 2382.0 Million by 2032, exhibiting a growth rate (CAGR) of 12.6% during 2024-2032.
- North America leads the market, accounting for the largest single-use medical device reprocessing market share.
- Based on the application, the market has been divided into general surgery, anesthesia, arthroscopy and orthopaedic surgery, cardiology, gastroenterology, gynaecology, urology, and others.
- Hospitals account for the majority of the market share owing to the increasing number of patients seeking effective healthcare services.
- The rising need for cost reduction in healthcare services is impelling the growth of the market.
- Environmental sustainability is becoming a priority in various sectors, including healthcare, driving the need for single-use medical devices.
Industry Trends and Drivers:
- Cost Efficiency and Budget Constraints in Healthcare Systems:
The rising need for cost reduction in healthcare services is impelling the growth of the market. Reprocessing of single-use medical devices (SUDs) involves the cleaning, disinfecting, and sterilizing of devices originally intended for one-time use to ensure they are safe for reuse. This process can lead to substantial cost savings for healthcare facilities, which is particularly crucial as these institutions face constant pressure to manage budgets effectively. Moreover, reprocessed devices can cost less than their new counterparts, allowing hospitals and clinics to allocate resources more efficiently elsewhere, such as toward patient care or the adoption of advanced technologies.
- Environmental Concerns and Sustainability Practices:
Environmental sustainability is becoming a priority in various sectors, including healthcare, driving the need for single-use medical devices. Reprocessing helps reduce medical waste significantly, which is critical given the large volume of waste generated by healthcare facilities annually. By reusing devices, hospitals can minimize their environmental footprint, decreasing the amount of non-biodegradable waste deposited in landfills and reducing the need for new raw materials to manufacture more devices. Furthermore, this practice aligns with global initiatives aimed at promoting sustainability in healthcare by not only managing waste but also conserving energy and water used in the production and disposal of medical devices.
- Regulatory Support and Advancements in Reprocessing Technologies:
The expansion of the market is also supported by favorable regulatory frameworks established by healthcare authorities worldwide. Regulatory bodies have set stringent guidelines and standards for the reprocessing of SUDs to ensure they meet safety and performance criteria akin to new devices. These regulations are bolstering the confidence of healthcare providers in using reprocessed devices. Additionally, advancements in reprocessing technology are improving the efficiency and safety of reprocessed devices, making them more appealing to healthcare facilities.
Request for a sample copy of this report: https://www.imarcgroup.com/single-use-medical-device-reprocessing-market/requestsample
Single-use Medical Device Reprocessing Market Report Segmentation:
By Device Type:
- Class I Devices
- Laparoscopic Graspers
- Scalpels
- Tourniquet Cuffs
- Other Class I Devices
- Class II Devices
- Pulse Oximeter Sensors
- Sequential Compression Sleeves
- Catheters and Guidewires
- Other Class II Devices
Class II devices represent the largest segment as they allow healthcare facilities to significantly reduce procurement costs.
By Application:
- General Surgery
- Anesthesia
- Arthroscopy and Orthopaedic Surgery
- Cardiology
- Gastroenterology
- Gynaecology
- Urology
- Others
Based on the application, the market has been divided into general surgery, anesthesia, arthroscopy and orthopaedic surgery, cardiology, gastroenterology, gynaecology, urology, and others.
By End User:

- Hospitals
- Ambulatory Surgical Centers
- Others
Hospitals account for the majority of the market share owing to the increasing number of patients seeking effective healthcare services.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America’s dominance in the single-use medical device reprocessing market is attributed to the rising focus on environmental sustainability and waste reduction.
Top Single-use Medical Device Reprocessing Market Leaders:
The single-use medical device reprocessing market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies. Some of the key players in the market are:
- Arjo Inc.
- Innovative Health
- Johnson & Johnson
- Medline Industries LP
- NEScientific Inc.
- Steripro Canada
- Stryker Corporation
- SureTek Medical
- Vanguard AG
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145